Gene Editing’s Promise Curing Sickle Cell
Understanding Sickle Cell Disease
Sickle cell disease (SCD) is a debilitating inherited blood disorder affecting millions worldwide. It’s caused by a single point mutation in the gene responsible for producing hemoglobin, the protein in red blood cells that carries oxygen. This faulty hemoglobin causes red blood cells to become rigid and sickle-shaped, leading to a cascade of problems. These misshapen cells clog blood vessels, causing intense pain (sickle cell crises), organ damage, and a shortened lifespan. Current treatments largely focus on managing symptoms, with bone marrow transplants offering a potential cure but limited by donor availability and significant risks.
Gene Editing: A Revolutionary Approach
Gene editing technologies, particularly CRISPR-Cas9, offer a groundbreaking approach to tackling SCD at its genetic root. CRISPR acts like molecular scissors, precisely targeting and correcting the faulty gene responsible for the disease. This targeted gene repair offers the potential for a functional cure, eliminating the need for lifelong symptom management and improving the quality of life for those affected. Unlike bone marrow transplants, this approach doesn’t require finding a compatible donor, making it potentially accessible to a wider range of patients.

How CRISPR-Cas9 Targets the Sickle Cell Gene
The CRISPR-Cas9 system works by utilizing a guide RNA molecule, designed to match a specific DNA sequence within the sickle cell gene. This guide RNA directs the Cas9 enzyme, a protein that acts as molecular scissors, to the precise location of the mutation. Once there, Cas9 cuts the DNA, creating a double-stranded break. The cell’s natural repair mechanisms then kick in, attempting to mend the break. This process can be further guided to incorporate a corrected version of the gene, replacing the mutated sequence with a healthy one, effectively correcting the genetic defect that causes SCD.
Ex Vivo vs. In Vivo Gene Editing
Gene editing for SCD can be approached in two main ways: ex vivo and in vivo. Ex vivo editing involves removing a patient’s hematopoietic stem cells (HSCs) – the cells that give rise to all blood cells – from their bone marrow. These cells are then edited in a laboratory setting using CRISPR-Cas9, correcting the faulty gene. Once the corrected cells have multiplied sufficiently, they are infused back into the patient’s bloodstream, where they can regenerate healthy red blood cells. In vivo gene editing, on the other hand, involves delivering the CRISPR-Cas9 system directly into the patient’s body, where it targets and corrects the faulty gene within the body’s own HSCs. This method avoids the need for cell harvesting and reinfusion but presents its own challenges in terms of targeted delivery and off-target effects.
Clinical Trials and Promising Results
Several clinical trials are underway using both ex vivo and in vivo approaches to gene editing for SCD. Early results from these trials have been incredibly encouraging, demonstrating significant improvements in patients’ health. Many participants have seen a reduction or elimination of painful vaso-occlusive crises, a hallmark symptom of SCD. Furthermore, studies are showing increases in the levels of fetal hemoglobin, a type of hemoglobin that can compensate for the faulty adult hemoglobin, providing further relief from SCD symptoms. These results represent a major leap forward in the fight against this devastating disease.
Challenges and Future Directions
Despite the remarkable progress, challenges remain. Ensuring the precise targeting of CRISPR-Cas9 to avoid off-target effects – unintended edits to the genome – is crucial to minimize potential risks. The cost of gene editing therapies is also a significant barrier to widespread accessibility, although prices are likely to decrease as the technology matures and becomes more widely adopted. Further research is focused on optimizing gene editing protocols, improving delivery methods, and reducing the overall cost of these potentially life-saving treatments. The development of safer and more efficient gene editing tools is also an ongoing area of intense research.
Ethical Considerations and Societal Impact
The development of gene editing technologies for treating SCD raises important ethical considerations. Discussions around equitable access to these potentially life-changing therapies are paramount. Researchers and policymakers must work together to ensure that these treatments are available to all who need them, regardless of their socioeconomic status or geographic location. The long-term effects of gene editing also need careful monitoring and evaluation. Open dialogue and transparent communication are vital to navigate the ethical implications of this powerful technology and to harness its potential for the benefit of humanity. Click here to learn about CRISPR gene editing and sickle cell.
CRISPR Explained A Simple Guide to Gene Editing
What is CRISPR-Cas9?
CRISPR-Cas9 is a revolutionary gene-editing tool that’s transforming the fields of biology and medicine. Imagine being able to precisely cut and paste DNA, the instruction manual of life. That’s essentially what CRISPR-Cas9 allows us to do. It’s a naturally occurring system found in bacteria, adapted and refined by scientists to be a highly precise and efficient gene editing tool. The “CRISPR” part is a type of DNA sequence found within the bacteria’s genome, acting like a memory bank of past viral infections. “Cas9” refers to an enzyme, a protein that acts like molecular scissors, capable of cutting DNA at specific locations. This combination allows for targeted modifications to DNA.
How Does CRISPR Work?
Think of it like a guided missile targeting a specific location on a vast landscape. First, scientists design a short RNA molecule (a type of genetic material similar to DNA) that’s complementary to the DNA sequence they want to target. This RNA acts as a guide, leading the Cas9 enzyme to the exact spot on the genome where the modification needs to happen. Once the Cas9 enzyme arrives at the target location, it acts as molecular scissors, making a precise double-stranded break in the DNA. The cell’s natural repair mechanisms then kick in, and this is where the magic happens. Scientists can either let the cell repair the break naturally (often resulting in a small insertion or deletion, effectively disrupting the gene) or they can provide a DNA template for the cell to use in repairing the break, inserting a new gene or correcting a faulty one.
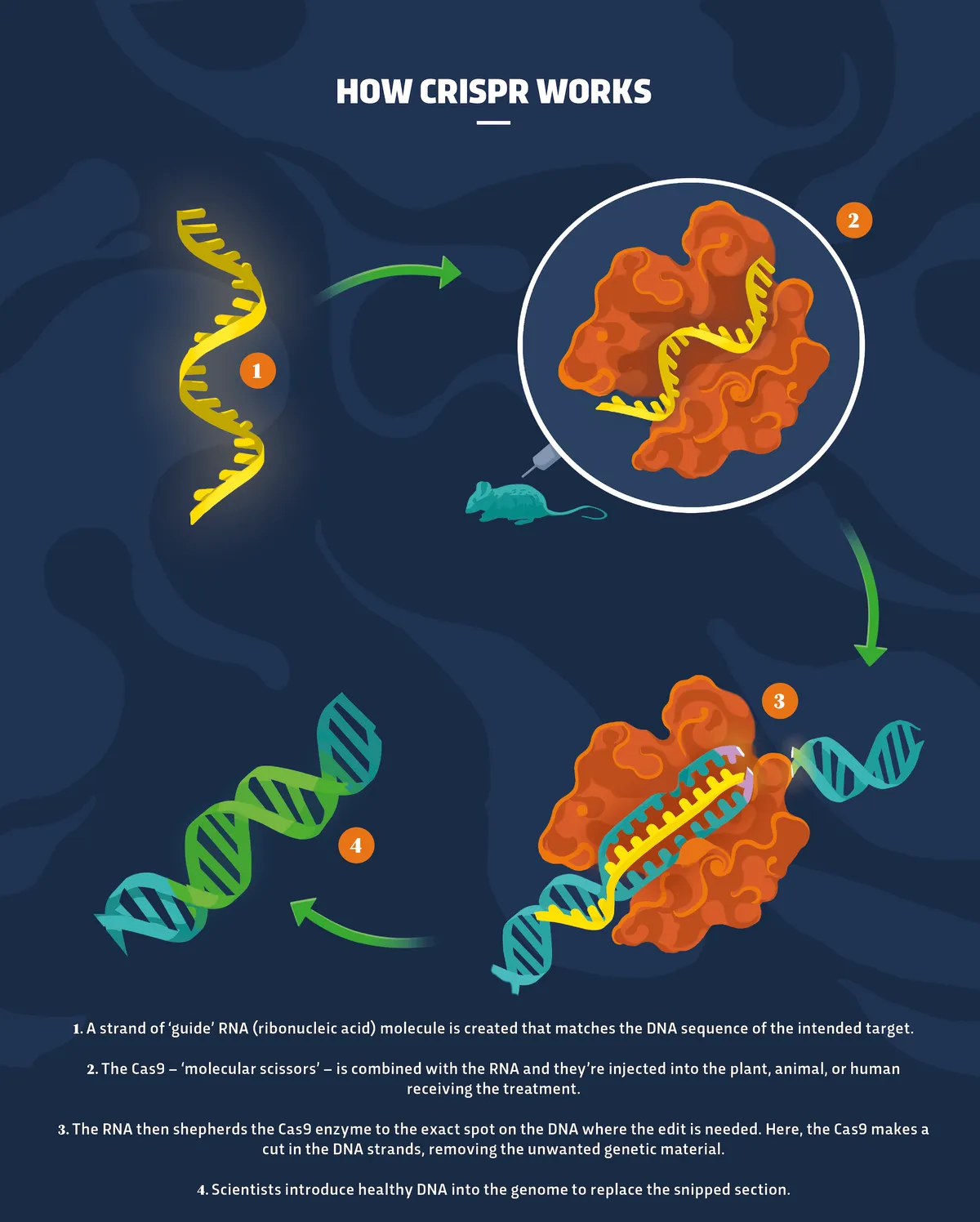
The Precision of CRISPR
One of the most remarkable aspects of CRISPR-Cas9 is its precision. Unlike older gene editing methods, which often resulted in unintended changes to the genome, CRISPR offers a significantly higher level of accuracy. The guide RNA ensures that the Cas9 enzyme cuts the DNA at the intended location, minimizing off-target effects. While off-target edits are still a possibility, researchers are continually developing methods to improve the specificity of CRISPR and reduce the risk of unintended consequences. This precision is what makes CRISPR so powerful for research and potential therapeutic applications.
Applications of CRISPR Technology
The potential applications of CRISPR are vast and far-reaching. In research, it’s revolutionizing our understanding of gene function and disease mechanisms. Scientists use CRISPR to create animal models of human diseases, allowing them to study disease progression and test potential treatments. In medicine, CRISPR holds enormous promise for treating genetic diseases. Researchers are exploring the use of CRISPR to correct genetic defects responsible for conditions like cystic fibrosis, sickle cell anemia, and Huntington’s disease. Beyond human health, CRISPR has applications in agriculture, allowing scientists to develop crops that are more resistant to pests, diseases, and harsh environmental conditions. It also holds potential for enhancing food production and addressing global food security challenges.
Ethical Considerations and Challenges
Despite its immense potential, CRISPR technology also raises important ethical considerations. The ability to alter the human germline (the genes passed down from generation to generation) raises concerns about unintended consequences for future generations. Many scientists advocate for a cautious approach to germline editing, emphasizing the need for thorough research and careful ethical debate before proceeding with such applications. Furthermore, the accessibility and affordability of CRISPR technology are also important considerations. Ensuring equitable access to this powerful tool is crucial to prevent its misuse and to ensure that its benefits are shared broadly.
The Future of CRISPR
CRISPR-Cas9 technology is still relatively new, but its rapid development and diverse applications suggest a bright future. Researchers are continually refining the system, improving its precision, and expanding its capabilities. New variations of CRISPR are being developed, offering even greater accuracy and versatility. While challenges remain, the potential of CRISPR to revolutionize various fields is undeniable. From treating genetic diseases to developing sustainable agriculture, CRISPR is poised to shape the future of medicine, science, and technology in profound ways.
CRISPR Beyond Cas9: Other CRISPR Systems
While Cas9 is the most well-known CRISPR-associated enzyme, other systems are being explored and developed. These different Cas enzymes have unique properties that may be better suited for specific applications. Some offer improved precision, while others can perform different types of gene editing, like base editing (changing a single DNA base without causing a double-strand break). This diversity expands the toolbox for gene editing, opening doors to even more complex and precise manipulations of the genome.
Overcoming Limitations and Improving Efficiency
Current research focuses on enhancing CRISPR’s efficiency and reducing off-target effects. Scientists are developing improved guide RNA designs, exploring alternative Cas enzymes, and using innovative delivery methods to enhance the effectiveness of CRISPR therapies. This continuous improvement will ultimately make CRISPR a more robust and reliable tool for gene editing, unlocking further possibilities for its therapeutic and research applications. The
CRISPR’s Promise Curing Inherited Diseases
Understanding CRISPR-Cas9: A Gene-Editing Revolution
CRISPR-Cas9, often shortened to CRISPR, has emerged as a groundbreaking gene-editing tool with the potential to revolutionize medicine. This technology allows scientists to precisely target and modify specific sections of DNA, offering a powerful approach to treating a wide range of diseases, particularly those with a genetic basis. Unlike previous gene therapy methods, CRISPR’s accuracy and relative simplicity have made it a frontrunner in the race to cure inherited disorders. At its core, CRISPR works like a highly sophisticated molecular scissor, cutting DNA at a predetermined location, allowing scientists to then either disable a faulty gene or insert a corrected version.
Inherited Diseases: A Target for CRISPR
Inherited diseases, caused by mutations in our genes, affect millions worldwide. These conditions can range from relatively mild to severely debilitating and life-threatening. Many such diseases lack effective treatments, leaving patients with limited options. Conditions like cystic fibrosis, sickle cell anemia, Huntington’s disease, and muscular dystrophy are all prime candidates for CRISPR-based therapies. The ability to correct the underlying genetic defect offers a potential cure rather than simply managing the symptoms, representing a paradigm shift in how we approach these illnesses.

How CRISPR Works in Treating Inherited Diseases
The process begins with identifying the specific gene mutation responsible for the disease. Scientists then design a guide RNA molecule that will bind to this specific DNA sequence. This guide RNA acts as a GPS, directing the Cas9 enzyme (the “molecular scissor”) to the precise location on the DNA. Once there, Cas9 cuts the DNA, creating a double-strand break. The cell’s natural repair mechanisms then kick in, either repairing the break using a provided template DNA (containing the correct gene sequence) or disabling the faulty gene altogether. This precise manipulation holds the key to correcting genetic errors and potentially curing the inherited disease.
Current Clinical Trials and Promising Results
While still in its relatively early stages, CRISPR technology is rapidly advancing. Several clinical trials are underway, testing the effectiveness and safety of CRISPR-based therapies for various inherited diseases. Early results from some trials are encouraging, showing promising outcomes in treating conditions like beta-thalassemia and sickle cell anemia. These initial successes provide strong evidence supporting the therapeutic potential of CRISPR and highlight the rapid progress being made in translating this technology from the laboratory to the clinic. However, it’s crucial to remember that these are early findings, and more research and trials are needed to fully understand the long-term effects and safety profile.
Challenges and Ethical Considerations
Despite the tremendous promise, CRISPR therapy is not without its challenges. Off-target effects, where the CRISPR system inadvertently modifies unintended parts of the genome, remain a significant concern. Scientists are actively working to improve the specificity and accuracy of the technology to minimize this risk. Furthermore, ethical considerations surrounding germline editing (modifying genes that can be passed down to future generations) are a subject of intense debate. The potential for unintended consequences and societal implications necessitates careful consideration and robust regulatory frameworks.
The Future of CRISPR in Gene Therapy
The future looks bright for CRISPR technology in treating inherited diseases. Continued research and development will likely address many of the current challenges, improving the accuracy, efficiency, and safety of CRISPR-based therapies. As our understanding of the human genome grows and technology improves, we can expect to see an increasing number of clinical trials exploring CRISPR’s potential for a wider range of inherited disorders. The development of more sophisticated delivery methods and the refinement of CRISPR systems will further enhance the effectiveness and accessibility of these treatments. CRISPR holds the potential to transform the landscape of gene therapy, providing hope for millions affected by debilitating inherited diseases.
Beyond Inherited Diseases: Expanding Horizons
The applications of CRISPR extend beyond inherited diseases. Researchers are exploring its use in treating acquired diseases like cancer, viral infections, and autoimmune disorders. The ability to precisely modify genes opens up new avenues for therapeutic intervention in a wide range of conditions. This versatility makes CRISPR a truly transformative technology with the potential to impact human health on a global scale. While challenges remain, the rapid progress and unwavering dedication of researchers promise a future where many currently incurable diseases become manageable or even curable. Learn more about CRISPR gene editing technology here.

